4-1 Mineral Resources
The importance of Earth’s mineral resources to our technology-rich lives is summarized in section 8.0 of the textbook. Almost everything we touch and use during the day is extracted from the Earth. Think about your day so far. Perhaps you started with a toothbrush made of plastic, or you sat on the plastic seat of a porcelain toilet, or had a shower with water made hot by energy from the Earth. Much of what you got dressed in may be made of synthetic fibres, derived from petroleum. You may then have had coffee from a pottery mug (made from clay), or water or juice from a glass (made from sand) and eaten from a pottery dish using a metal spoon or fork. If you went to work or school you possibly rode in a metal car or bus running on fossil fuel, or on a metal bicycle, along a road made of concrete or asphalt. Right now, you may be sitting in a chair made of synthetic material, and you might be reading this from an electronic device, like the one illustrated on Figure 8.0.1, that is powered by energy from the Earth, and is also dependent on batteries made of a wide variety of Earth resources (see Figure 8.0.3).
Section 8.1 covers our use of metals and where get them. Ordinary rock has very little recoverable metal in it—typical ores have thousands of times higher concentrations—so some efficient concentrating processes must have taken place in order to make an ore deposit. Some of those are described in the rest of section 8.1; most involve heat from the Earth’s interior, as you’ll see when you complete Exercise 8.2 The Importance of Heat and Heat Engines.
As long as they stay in the ground, metallic ores don’t have much of an environmental impact, but when we bring them to surface and break them into small pieces and then grind them to a powder they can react quickly and cause all sorts of problems. As described in section 8.2, the real culprit is the mineral pyrite (FeS2) because it oxidizes readily under surface conditions (especially when ground up), and then releases acid into the environment. The acidity is an environmental problem by itself, but that is exacerbated because heavy metals are particularly soluble in acidic water, and so can reach toxic concentrations (see Box 8.1 Acid Rock Drainage at Mt. Washington, BC). Some of the other environmental risks from mining are summarized in Table 8.2.1.
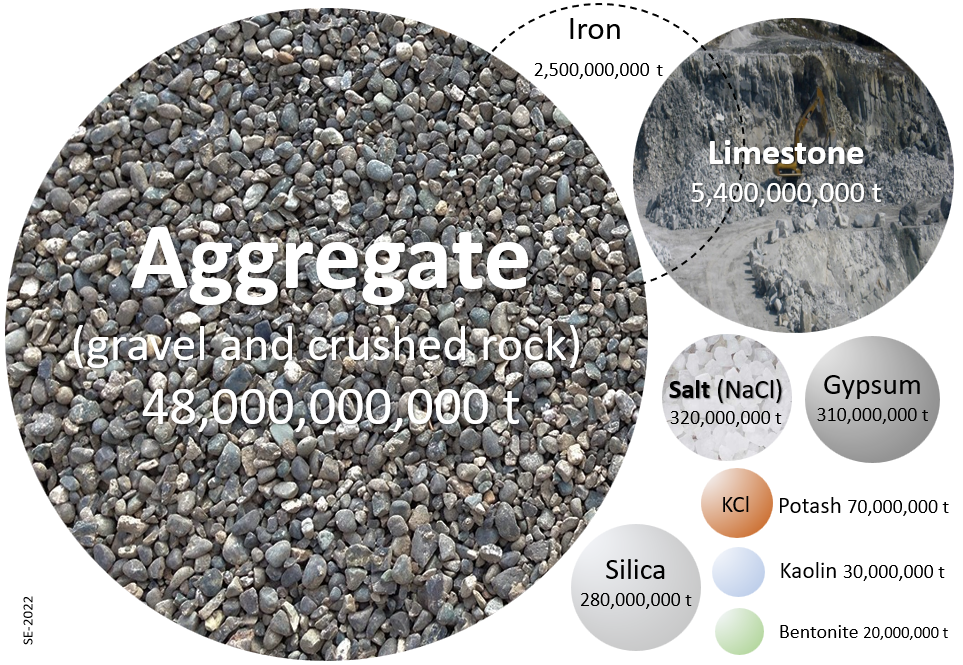
Industrial materials are, by far, the things that we dig up the most. As shown on Figure 4-1 below, aggregate is the main resource, and in fact we produce more aggregate than all other resources combined (including all metals and all fossil fuels). Most of it is used to make concrete and roads. Limestone is the next most common. Most of that is used as an ingredient of concrete, and for that it goes first through the calcination reaction, which requires heat that usually comes from a fossil fuel:
CaCO3 (calcite) + heat -> CaO (lime for cement) + CO2
The emissions from the fossil fuel heat source and the CO2 from calcination represent about 8% of our total CO2 emissions.
Exercise 4-1 Sources of Important Lighter Metals
Please complete Exercise 8.3 Sources of Important Lighter Metals in the Environmental Geology textbook.
Section 8.4 of the textbook provides an overview of the formation and occurrence of fossil fuels. Since the fossil-fuel era is nearly over (and has to be over if we are going to continue to live on this planet), we don’t need to dwell on that information. Figure 4-2 below shows the tonnage of fossil fuels used globally in 2020. Coal makes up the greatest tonnage (more than oil and gas combined). Reducing our production and use of coal is the most important step that we can take because coal emits the most CO2 per unit of energy produced, and many times more of the other pollutants that are making cities in coal-burning areas difficult—and even dangerous—to live in. Some of those pollutants include nitrogen-oxides, sulphur dioxide, fine particular matter, mercury, and volatile organic compounds (e.g., benzene, toluene, ethylbenzene, and xylene).

Section 8.5 of the textbook provides an overview of the climate and other environmental implications of our use of Earth resources. The largest and most significant are the climate-change implications of fossil fuels, but our prolific use of other resources (metals, concrete etc.) also has serious climate and other consequences that we should think about every time we buy something new, even something small, such as a new mobile phone.
As shown on Figure 9.0.2 in the textbook, we are slowly starting to move away from fossil fuels, but we have a long way to go, and to meet our aspirations to limit global warming to 1.5° C, we need to start moving faster.

